
OUR RESEARCH
Techniques we use to study developmental pathology of neuropsychiatric diseases:
Patient iPSC-derived cerebral organoids
Cell sorting
2-photon Calcium imaging
Multielectrode arrays
Proteomics and single-cell RNA sequencing
Cell transplantation
Stereological cell counting
Electrophysiology (E-LTP, L-LTP and LTD)
Behavioral assays

Footprint of more than 400 organoids

cerebral organoid
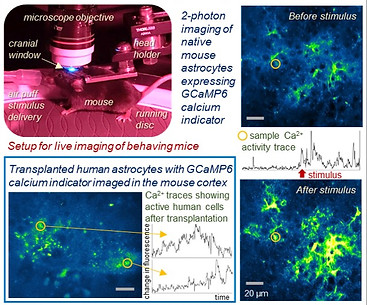
GFP-positive human astrocyte transplantation into neonatal mouse cortex, imaged 2 months post-transplantation

Studying developmental pathology of neuropsychiatric diseases: Cognitive dysfunction is a core feature of neuropsychiatric as well as neurodegenerative diseases. We are interested in understanding the cellular mechanisms underlying the cognitive dysfunction seen in diseases such as autism and schizophrenia. Existing animal models do not capture the genetic heterogeneity of these polygenetic diseases, and human postmortem studies offer very limited insight into the time course of disease progression. Patient-derived induced pluripotent stem cells (iPSCs) provide the best experimental system to identify the underlying molecular and cellular defects in polygenic diseases. iPSCs can be manipulated to form brain-like structures termed cerebral organoids. This culture system recapitulates the spontaneous generation of major brain cell types, providing a unique opportunity to study molecular and cellular abnormalities using patient cerebral organoids. We use cerebral organoids derived from patient iPSCs in combination with cell sorting, transplantation, electrophysiology and mouse behavioral assays to understand underlying mechanisms of defective synaptic plasticity and cognitive function in autism and schizophrenia.
Autism: We employ control and patient cerebral organoids to spontaneously generate disease astrocytes. Cell fate specification is temporally regulated in cortical development and is defined by the sequential appearance of neurons and glia. Cerebral organoids recapitulate the temporal sequence of cortical development seen in developing embryos. Importantly, the organoid system also allows us to populate disease astrocytes in an environment that mimics the early brain pathology. Therefore, cerebral organoids are likely to serve as a more representative system to preserve disease-specific features in patient astrocytes compared to directed differentiation protocols.
Schizophrenia. Schizophrenia has a common onset in early adulthood, however evidence has accumulated for several decades that schizophrenia has much earlier developmental origins. Because the collection of primary human neural tissue from cases prior to onset remains ethically and technically impossible, very limited insight has been gained as to when schizophrenia pathology truly begins. To overcome this, we use 3D stem cell organoid systems. By conducting high-content deep phenotyping screens of patient-derived organoids, we are determining cell-specific neuropathology for
schizophrenia.
OUR LAB

Dilek Colak, PI: Dilek is an Assistant Professor in the Brain and Mind Research Institute (BMRI). She earned her PhD degree from the Ludwig Maximilian University in Munich, Germany. Her PhD studies showed that neurogenesis in adult neural stem cells can be initiated upon inhibition of the apparent default pathway that is oligodendrogenesis. During her PhD, Dilek was also actively involved in projects that aimed to re-instruct neurogenesis after brain injury. During her postdoc studies in New York, she explored the physiological role of local mRNA translation. Her study identified RNA degradation pathway nonsense-mediated mRNA decay (NMD) as a mechanism that regulates axon guidance in vivo. During this time, she also became interested in the mechanism of FMR1 gene silencing in Fragile X Syndrome (FXS), which is a trinucleotide repeat expansion disease and the most common monogenic cause of autism. Using FXS human embryonic stem cells, she discovered that the expanded repeats of the FMR1 mRNA interacts with the genomic DNA that then triggers FMR1 promoter repression. Her studies showed for the first time that a coding RNA could bind DNA to induce epigenetic silencing.
email: dic2009@med.cornell.edu

Michael Notaras, Postdoctoral fellow: Michael comes from Australia and holds a PhD in neuroscience from the University of Melbourne, Australia, where he studied the role of a neurotrophin coding polymorphism in sensitivity to stress and stress-induced brain remodelling. His work has led to five first author publications, including two in Molecular Psychiatry (Notaras et al., Molecular Psychiatry 2015; Notaras et al., Molecular Psychiatry 2016). He joined the Colak laboratory in 2016 to obtain postdoctoral training in the use of induced-pluripotent stem cells, 3D organotypic culturing methods, transplantation, microfluidic devices, and synaptic biology. Michael’s primary academic interest is the neurobiology of mental disorders, and he currently works on projects that seek to elucidate the mechanisms of brain aberrations that lead to schizophrenia and autism. When not in the laboratory, you may see Michael exploring New York’s food scene or find him writing in one of the many cafés on the Upper East Side of Manhattan.
email: mjn2004@med.cornell.edu

Megan Allen, Postdoctoral fellow: Megan recently finished her PhD studies in Georgetown University, Washington-DC, in the neuroscience program. Her work revealed that the protease MMP-1 regulates synaptic plasticity through increases in intracellular Ca2+ concentrations and dendritic arborization in hippocampal neurons in vitro and in vivo (Allen et al., Scientific Reports 2016). In addition to her first author paper, she has published five co-author papers during her PhD studies. Megan is currently working on projects that explore the role of glial cells in idiopathic autism. The techniques she mastered make her an excellent fit to the lab: Primary hippocampal cultures, receptor internalization, live cell Calcium imaging, SDS-PAGE, dendritic Sholl analysis, in vivo spine analysis, and network activity recording with multielectrode arrays.


Nicole Volk, Research technician: Nicole received her BS degree in Biochemistry at Rutgers, State University of New Jersey. During her undergrad research assistant studies, she studied post-transcriptional regulatory elements of neocortical neurogenesis; specifically focusing on RNA binding proteins CUGBP1 and HuD. During her first laboratory technician appointment, and expanding upon her undergraduate studies, Nicole investigated the role of mRNA decapping enzymes, DcpS and Dcp2, in neocortical neurogenesis. She joined the Colak laboratory in May 2016. In addition to routinely preparing microfluidic devices, Nicole is responsible for ordering, perfusion, tissue sectioning, maintaining mouse colonies and genotyping.
email: niv2001@med.cornell.edu
email: taj4002@med.cornell.edu
Aiman Lodhi, research volunteer: Aiman is an undergrtad student in the Biochemistry Department of Hunter College, NY. To broaden her knowledge and skills in neuroscience, she has been volunteering in our lab since August 2018 . Aiman performs cryosectioning of cerebral organoids as well as stereological countings in mice transplanted with human astrocytes.
email: ail4001@med.cornell.edu
Former members

Nicole Sayles, rotation student

Shellie Ann Dick, rotation student

Jacques Lara, research volunteer
NEWS
July 30, 2018, Dilek is giving a talk for the Department of Neurosurgery Grand Rounds at WCM
June 29, 2018, Mike is giving a talk for the BMRI Progress Report Seminar Series at MSKCC
February 2018, Dilek received her first R01
January 2018, Mike received the NHMRC CJ Martin Biomedical Fellowship
Dec 12, 2017, Mike is giving a talk for the Neurodevelopmental Group Meeting at MSKCC
April 21, 2016, Dilek is giving a talk at the Leon Levy Symposium at Columbia University
February 2016, Dilek received the Leon Levy Foundation Junior Investigator Grant
GALLERY


Awake animal Calcium imaging of transplanted astrocytes

Schematic of custom tripartite microfluidic device used in the study of synaptic events. This microfluidic device contains three channels separated by microgrooves (M), which creates both fluidic and physical isolation between channels. When neurons are cultured in channels 1 and 3, channel 2 (middle channel) becomes enriched with dendrites and axons. As the microgrooves that separate channels 1 and 3 from the middle channel are of different lengths, dendrites of neurons cultured only in channel 1 (postsynaptic) can extend to the synaptic channel located in the middle of the device. At DIV21, immunostaining for the synaptic marker SV2 shows that the synaptic channel is enriched with synapses.
RNA regulation in synaptic plasticity and cognitive function: Local mRNA translation has been implicated in synaptogenesis, synaptic plasticity as well as learning and memory. Most work in this field has focused on understanding the pathways that activate mRNA translation in dendrites. We are interested in exploring the role of mRNA degradation pathways in synaptic regions and their role in synaptic plasticity in normal and disordered state. mRNA degradation pathways have been linked to various neurodevelopmental diseases including mental retardation, autism and schizophrenia. To determine the local function of mRNA degradation in dendrites, we use a novel microfluidic device to uniquely compartmentalize synapses. To study the contribution of the mRNA-degradation pathways into synaptic plasticity and cognitive function, we employ genetic mouse models in combination with electrophysiology experiments and behavioral tests.
MAP2 and TAU stainings, neurons in a tripartite device

Presynaptic channel
Postsynaptic channel
OUR LAB

Dilek Colak, PI: Dilek is an Associate Professor in the Brain and Mind Research Institute (BMRI). She earned her PhD degree from the Ludwig Maximilian University in Munich, Germany. Her PhD studies showed that neurogenesis in adult neural stem cells can be initiated upon inhibition of the default pathway that is oligodendrogenesis. During her PhD, Dilek was also actively involved in projects that aimed to re-instruct neurogenesis after brain injury. During her postdoc studies in New York, she explored the physiological role of local mRNA translation. Her study identified RNA degradation pathway nonsense-mediated mRNA decay (NMD) as a mechanism that regulates axon guidance in vivo. During this time, she also became interested in the mechanism of FMR1 gene silencing in Fragile X Syndrome (FXS), which is a trinucleotide repeat expansion disease and the most common monogenic cause of autism. Using FXS human embryonic stem cells, she discovered that the expanded repeats of the FMR1 mRNA interacts with the genomic DNA that then triggers FMR1 promoter repression. Her studies showed for the first time that a coding RNA could bind DNA to induce epigenetic silencing.
email: dic2009@med.cornell.edu

Michael Notaras, Postdoctoral fellow: Michael comes from Australia and holds a PhD in neuroscience from the University of Melbourne, Australia, where he studied the role of a neurotrophin coding polymorphism in sensitivity to stress and stress-induced brain remodelling. His work has led to five first author publications, including two in Molecular Psychiatry (Notaras et al., Molecular Psychiatry 2015; Notaras et al., Molecular Psychiatry 2016). He joined the Colak laboratory in 2016 to obtain postdoctoral training in the use of induced-pluripotent stem cells, 3D organotypic culturing methods, transplantation, microfluidic devices, and synaptic biology. Michael’s primary academic interest is the neurobiology of mental disorders, and he currently works on projects that seek to elucidate the mechanisms of brain aberrations that lead to schizophrenia and autism. When not in the laboratory, you may see Michael exploring New York’s food scene or find him writing in one of the many cafés on the Upper East Side of Manhattan.
email: mjn2004@med.cornell.edu

Megan Allen, Postdoctoral fellow: Megan recently finished her PhD studies in Georgetown University, Washington-DC, in the neuroscience program. Her work revealed that the protease MMP-1 regulates synaptic plasticity through increases in intracellular Ca2+ concentrations and dendritic arborization in hippocampal neurons in vitro and in vivo (Allen et al., Scientific Reports 2016). In addition to her first author paper, she has published five co-author papers during her PhD studies. Megan is currently working on projects that explore the role of glial cells in idiopathic autism. The techniques she mastered make her an excellent fit to the lab: Primary hippocampal cultures, receptor internalization, live cell Calcium imaging, SDS-PAGE, dendritic Sholl analysis, in vivo spine analysis, and network activity recording with multielectrode arrays.
email: mea2890@med.cornell.edu

Estibaliz Barrio Alonso, Postdoctoral fellow: Estibaliz comes from Spain and received her PhD from the Cajal Institute in Madrid. Her PhD studies focused on revealing how an aberrant re-entry in cell cycle by a differentiated neuron can affect its synaptic and functional properties. Her results showed that cell cycle reentry in neurons might contribute to cognitive impairment in early stages of Alzheimer’s disease and neuronal death susceptibility at later stages. Estibaliz has expertise in patch clamp technique. She joined the Colak lab in October 2019. Her studies focuses on the role of circadian rhythm proteins in synaptic plasticity and cognitive function.
email: esb4004@med.cornell.edu

Pablo Lituma, Postdoctoral fellow: Pablo obtained his PhD at the Albert Einstein College of Medicine in neuroscience in the laboratory of Dr. Pablo Castillo. During his PhD, in the Castillo lab, he spent many years training in electrophysiology and two-photon microscopy. His PhD studies have led to His PhD studies have led to five publications, three as first author, including one in Elife describing the functional role of presynaptic NMDA receptors in facilitating short-term plasticity and BDNF release at hippocampal mossy fiber synapses (Lituma et al., Elife 2021). Pablo also worked with microglia and investigated microglial regulation of synaptic function. Using transgenic mice, he characterized microglial activity, synaptic function and behavior. Specifically, he imaged microglia activity as well as measured synaptic transmission by whole-cell patch-clamp recording in acute hippocampal slices in Iba1-deficient (Aif1-/-) mice (Lituma et al., PNAS 2021). In our lab, Pablo uses both organoid system and mouse system to model ASD. His projects involve CRISPR gene deletions in organoids and conditional KO of candidate genes in mice that are followed by assessment of functional readouts including behavior, synaptic plasticity in slice cultures, in vivo calcium imaging, and microglia motility.
email: pjl4001@med.cornell.edu
.webp)
Nicolle Morey, Postdoctoral fellow: Nicolle obtained her PhD at the Macquarie University, NSW, Australia. During her PhD, in the van Ersel lab at the Dementia Research Centre, she explored the role of Tau in excitotoxicity and epileptogenesis. She has completed an extensive study where she generated, characterized, and treated a Dravet syndrome mouse model (Morey et al., Science Advances 2022). Her studies also involved other epilepsy disorders, childhood dementias (e.g., Schindler disease), FTD and MND. During these studies she mastered in brain organoid techniques including histological and functional characterization of forebrain organoids, Ca2+ imaging, microelectrode arrays, CRISPR gene editing, characterization of epilepsy mouse models by EEG implantation and recordings in adult mice, mouse behavioral assays, genotyping, immunostaining, Western blot, qPCR, and stereotaxic injections. Prior to her PhD studies, Nicolle worked at the Transgenic Animal Unit/Genome Editing, UNSW/Macquarie University as well as at the Genetic Manipulation Services (GeMs), Francis Crick Institute as a research scientist. Thus, she also has experience in murine embryo procedures, culture and manipulation of stem cells, mouse IVF, pronuclear microinjection in zygotes, and embryo transfers. Nicolle received many awards in the last ten years: Dementia Australia Research Foundation Grant (Associate Investigator, 2023-25; Macquarie University Post Graduate Research Fund travel grant, 2022; International Macquarie University Research Excellence Scholarship, 2020-23; Bath University Alumni Fund Scholarship, 2009-13). In our lab, Nicole uses both organoid system and mouse system to model ASD. Her projects involve CRISPR deletion of a high-risk ASD gene in organoids and conditional KO of the same gene in mice that are followed by assessment of phenotypic characterization.
email: nim4025@med.cornell.edu

email: iss4006@med.cornell.edu
Isidora Stankovic, Graduate student: Isidora was born and raised in Serbia. She completed her undergrad studies in biochemistry and neuroscience in Smith College, Northampton, MA. In 2018, as part of United Nations Foundations initiative, she served as a camp counselor for the first ever WiSci Girls STEAM Camp held in Prishtina, Kosovo. She has expertise in molecular cloning, RNA-Seq, Ribo-Seq, biochemical assays and protein mass spectrometry. Combining her molecular and neuroscience background, Isidora would like to study the effects of environmental risk factors and narcotics in cortical development at molecular and cellular level. She will use human cerebral organoid system as a model. Isidora is quite multicultural. In addition to English, she is also fluent in French, Spanish and Serbian. She has first-degree black belt in karate.


Natalie Wayland, Technician: Natalie received her BS degree in Biology, Summa Cum Laude with minors in Organic Chemistry, Ecology, and Neuroscience from Pace University in 2021. As an undergraduate student, she performed bioinformatic research to analyze RNA single cell sequencing data for genetic factors in sperm cell development and function in C. elegans. She utilized this work to complete and defend a thesis for the Pzorfheimer Honors College. She has had a great deal of research experience in genetics, plant biology, and microbial ecology. Natalie’s lab training formally began in her microbial ecology field research, where she published work regarding microbe soil composition following natural climate events, and expanded at Pace University, where she worked as the laboratory supervisor for the biology department. Her laboratory skills include, but are not limited to immunofluorescent staining, genotyping, DNA extraction and isolation, RNAi, and cell culturing. We trained Natalie in mouse behavioral phenotyping. She maintains mouse colonies and help with behavioral experiments.
email: naw4006@med.cornell.edu
Former members

Aiman Lodhi, technician

Nicole Sayles, rotation student

Tanya Jain, rotation student

Kelsy Lysek, technician


Shellie Ann Dick, rotation student
Jacques Lara, research volunteer

Careen Ford, rotation student

Aisha Irshad, summer internship
GALLERY












Use this link to view all our publications
https://www.ncbi.nlm.nih.gov/myncbi/DilekColak/bibliography/public/
SELECTED PUBLICATIONS
Allen, M., Huang, B.S., Notaras, M., Lodhi, A., Barrio Alonso, E., Wolujewicz, P., Witztum, J., Longo, F., Chen, Maoshan., Greening, D., Klann, E., Ross, E.M., Liston, C., Colak, D. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Molecular Psychiatry 2022 April 1. doi.org/10.1038/s41380-022-01486-x. PMID: 35365802. PMCID: PMC9135629.
Notaras, M., Lodhi, A., Dundar, F., Collier, P., Rodrick, T., Sayles, N., Tiglner, H., Greening, D., Colak, D. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol Psychiatry (2021). https://doi.org/10.1038/s41380-021-01316-6
Notaras, M., Lodhi, A., Fang, H., Greening, D., Colak, D. The Proteomic Architecture of Schizophrenia Cerebral Organoids Reveals Alterations in GWAS and Neuronal Development Factors. Translational Psychiatry (2021). https://doi.org/10.1038/s41398-021-01664-5
Notaras, M., Lodhi, A., Barrio-Alonso, E., Ford, C., Rodrick, T., Jones, D., Fang, H., Greening, D., Colak, D. Neurodevelopmental signatures of narcotic and neuropsychiatric risk factors in 3D human-derived forebrain organoids. Mol Psychiatry (2021). https://doi.org/10.1038/s41380-021-01189-9
Notaras, M., Allen, M., Longo, F., Volk, N., Toth, M., Jeon, N.L., Klann, E., Colak, D. UPF2 leads to degradation of dendritically-targeted mRNAs to regulate synaptic plasticity and cognitive function. Mol Pyschiatry doi: 10.1038/s41380-019-0547-5 (2019). PMID: 31636381
Deglincerti, A., Liu, Y., Colak, D., Hengst, U., Xu, G., Jaffrey, S.R. Coupled local translation and degradation regulate growth cone collapse. Nat Commun 6:6888 (2015).
Colak, D., Zaninovic, N., Cohen, M.S., Rosenwaks, Z., Yang, W.Y., Gerhardt, J., Disney, M.D., Jaffrey, S.R. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in Fragile X syndrome. Science 343:1002-5 (2014).
Gerhardt, J., Tomishima, M., Zaninovic, N., Colak, D., Yan, Z., Zhan, Q., Rosenwaks, Z., Jaffrey, S.R., Schildkraut, C.L. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol Cell 53:19-31 (2014).
Colak, D., Ji, S.-J., Porse B.T., Jaffrey, S.R. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 153:1252-65 (2013) (Previewed by Nicolas Preitner, Jie Quan and John G. Flanagan, Cell 153:1185-7).
Sirko, S., Behrendt, G., Johansson, P.A., Tripathi, P., Costa, M., Bek, S., Heinrich, C., Tiedt, S., Colak, D., Dichgans, M., Fischer, I.R., Plesnila, N., Staufenbiel, M., Haass, C., Snapyan, M., Saghatelyan, A., Tsai, L.H., Fischer, A., Grobe, K., Dimou, L., Götz, M. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 12:426-39 (2013).
Mira, H., Andreu, Z., Suh, H., Lie, C.D., Jessberger, S., Emeterio, J.S., Hortigüela, R., Marqués-Torrejón, M.A., Nakashima, K., Consiglio, A., Colak, D., Götz, M., Fariñas, I., and Gage, F.H. Signalling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7:78-89 (2010).
Jawerka, M., Colak, D., Dimou, L., Spiller, C., Lagger, S., Montgomery, R.L., Olson, E.N., Wurst, W., Göttlicher, M., Götz, M. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol 6:93-107 (2010).
Colak, D., Mori, T., Brill, M.S., Pfeifer, A., Falk, S., Deng, C., Monteiro, R., Mummery C., Sommer, L., Götz, M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci 28:434-46 (2008).
Buffo, A., Rite, I., Tripathi, P., Lepier, A., Colak, D., Horn, A.P., Mori, T., Götz, M. Origin and progeny of reactive gliosis - a novel source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A 105:3581-6 (2008).
Ma, L., Cantrup, R., Varrault, A., Colak, D., Klenin, N., Götz, M., McFarlane, S., Journot, L., Schuurmans, C. Zac1 functions through TGFbetaII to negatively regulate cell number in the developing retina. Neural Develop 2:11 (2007).
Buffo, A., Vosko, MR., Erturk*, D., Hamann, GF., Jucker, M., Rowitch, D., Götz, M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A 102:18183-8 (2005). * Erturk: Previously used last name.
